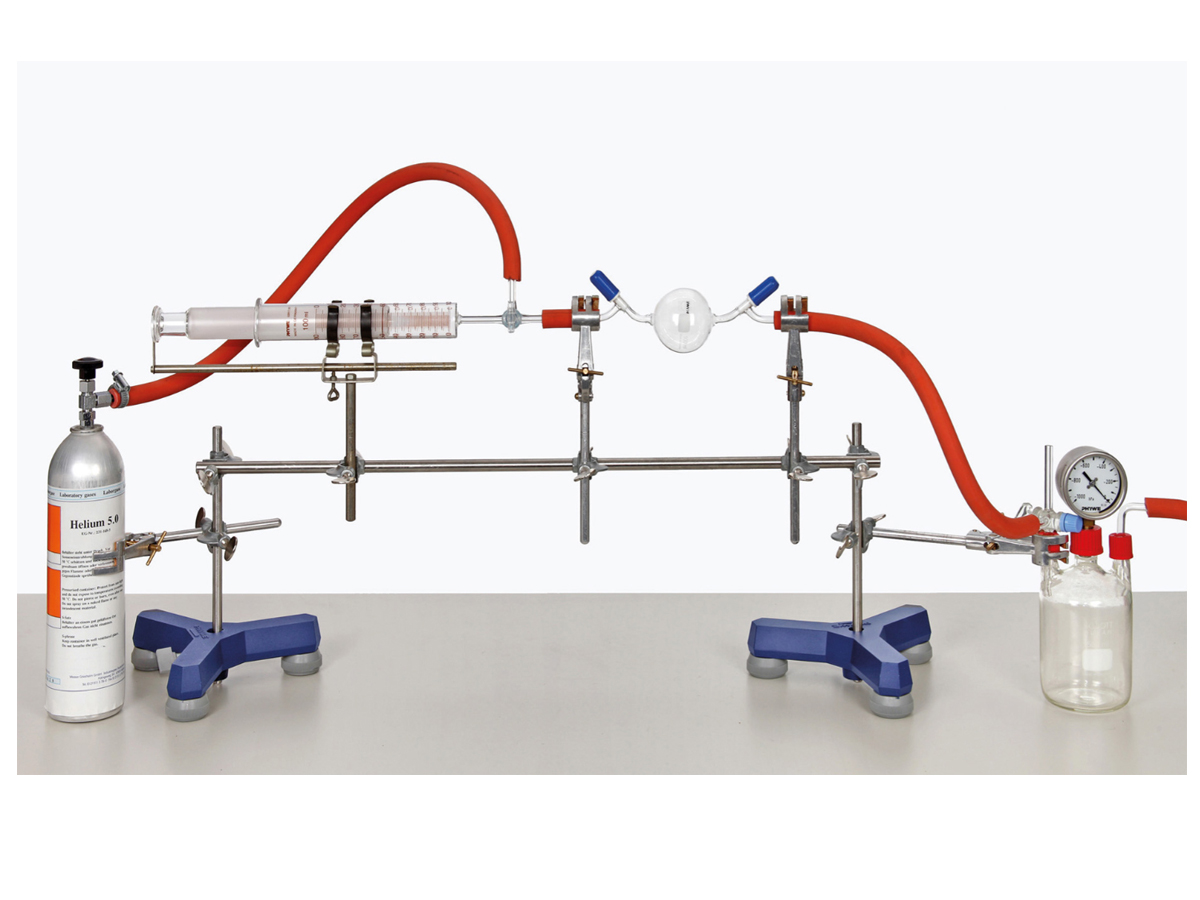
Determination of molar mass using the ideal gas law
ชุดทดลองหาค่ามวลโมลาร์โดยใช้กฎของแก๊สอุดมคติ
Article no. : P3010401
Principle
All gases may be considered, to a first approximation, to obey the ideal gas equation which relates the pressure p, volume V, temperature T and amount of substance n of a gas. The amount of gas n is expressed as the number of moles and is equal to m / M where m is the mass of gas present and M is the mass of one mole of the gas. The volume occupied by a known mass of gas is to be measured at a given temperature and pressure, so that the ideal gas equation can be used to estimate the molar mass of the gas.
Benefits
- Examination of many different gases possible
- Illustrative experimental setup
Tasks
- Determine the molar masses of the gases helium, nitrogen, carbon dioxide and methane.
Learning objectives
- Molar mass and relative molar mass
- Properties of gases
- Ideal and ordinary gases
- Equations of state


