Molten-salt electrolysis
ชุดทดลองอิเล็กโทรไลซิสเกลือหลอมเหลว
Article no. : P1310500
Principle
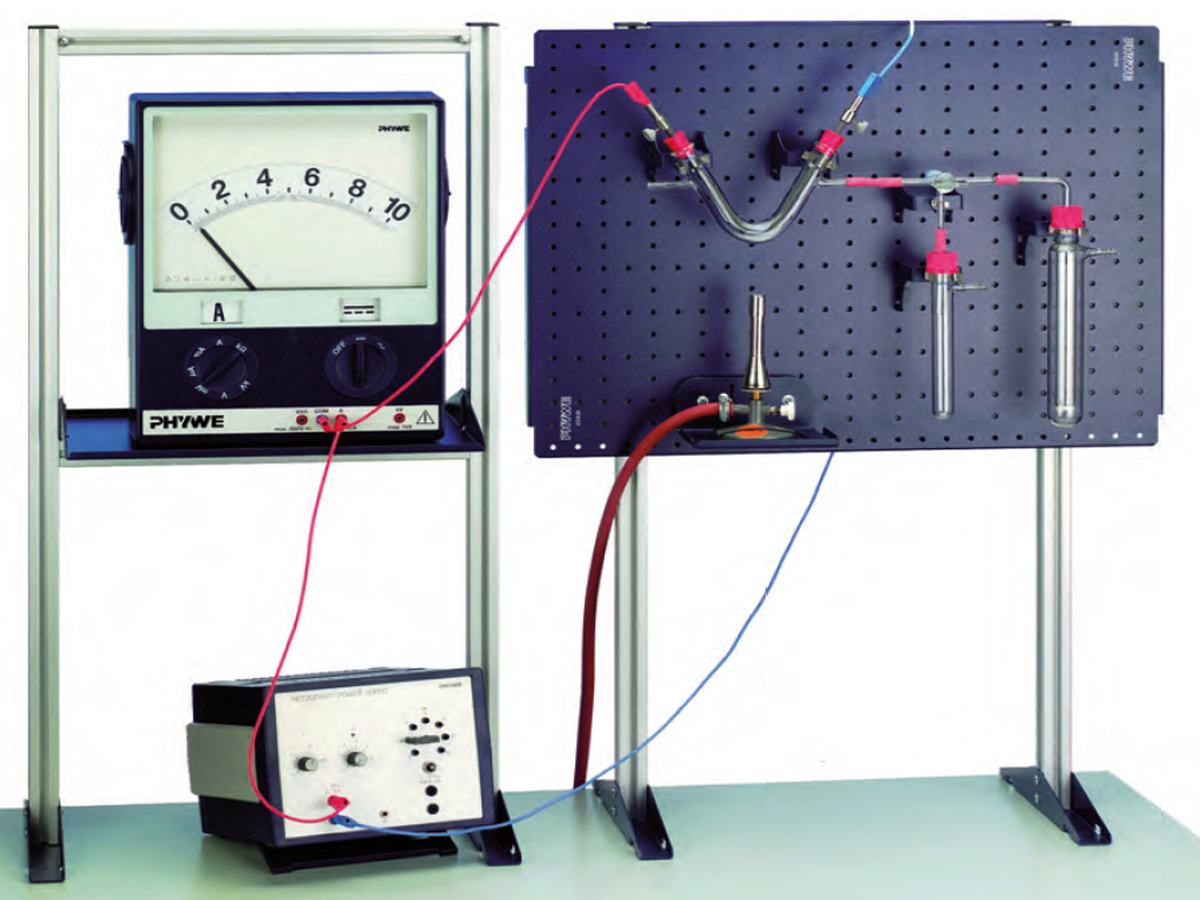
The electrolysis of molten sodium chloride to obtain chlorine and sodium, which can be used to produce sodium hydroxide, is an important industrial-scale process. The experiment is a simple demonstration of the important steps in this process. Due to the high melting point of sodium chloride, lead chloride (with a lower melting point) is used as starting material in the model experiment (instead of sodium chloride)
Benefits
- Easy and fast experiment set-up
- Impressive demonstration how to produce alkali metals like sodium
Tasks
Demonstration the electrolysis of molten sodium chloride to obtain chlorine and sodium.
Learning objectives
- Electrolysis
- Fused-salt electrolysis
- Preparation of Chlorine and sodium


